Why Pharma Companies Should File Patents Later In The R&D Process
This article was originally published in IAM Magazine on March 31, 2023.
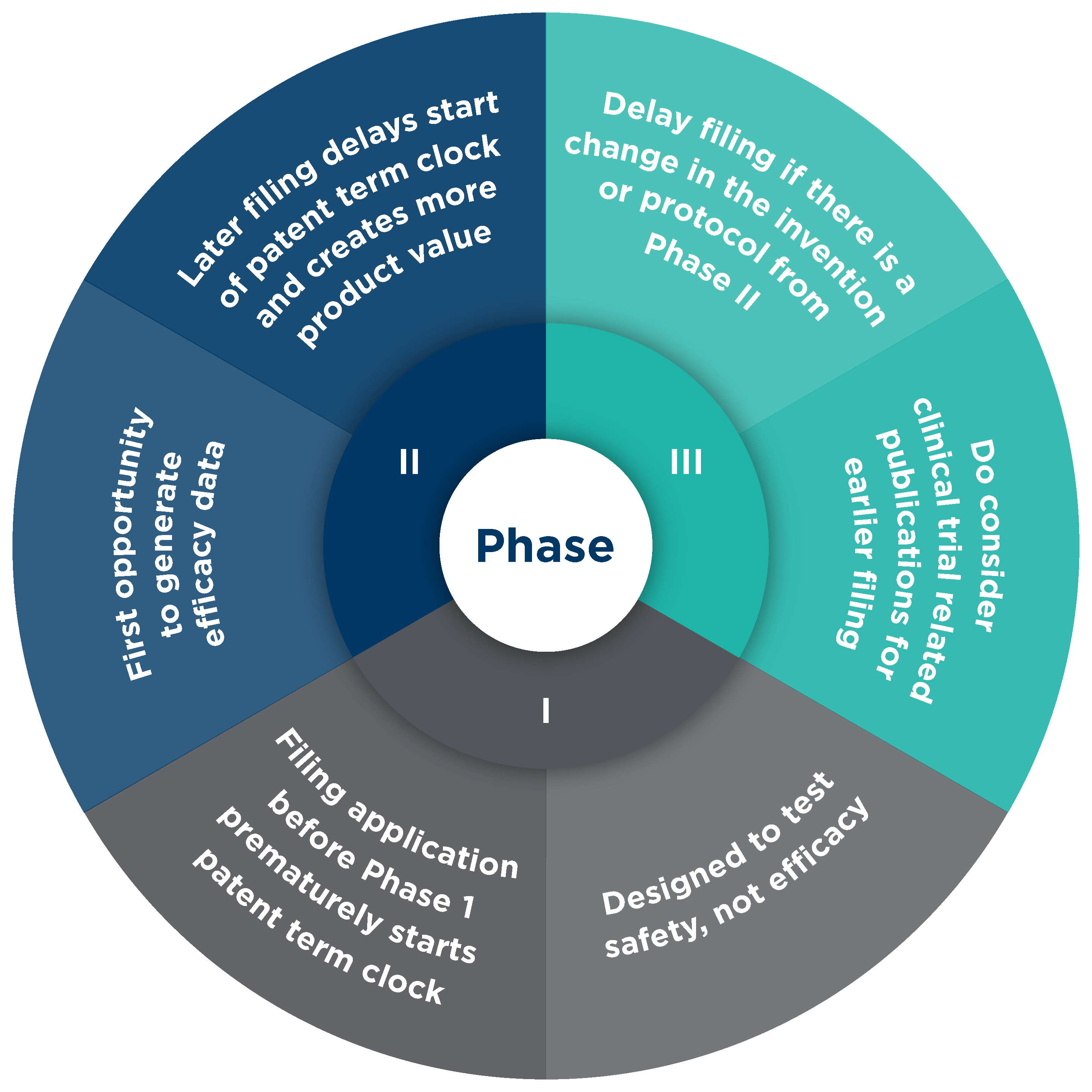
Clinical trial related patent applications are often filed prior to the start of the Phase I clinical trial to minimize the risk of invalidation of the resulting patent for public use (35 U.S.C. § 102(a)) based on the clinical trial itself. This conservative filing strategy is unnecessary for the majority of clinical trial-related inventions, and reduces the value of the patent by prematurely starting the patent term clock. Instead, the better default timeframe for filing such an application is after the start of the Phase II clinical trials, as the delay does not increase the risk of invalidation for public use, but maximizes the exclusivity and value provided by the patent.
A finding of public use requires the clinical trial demonstrate the invention works for its intended purpose (Pfaff v. Wells Elecs., 525 U.S. 55, 67-8 (1998); City of Elizabeth v. American Nicholson Pavement Co., 97 U.S. 126, 134 (1878)), and that the inventor did not exert sufficient control over the experimentation (Egbert v. Lippmann, 104 U.S. 333, 336 (1881)). Given the confidentiality agreements imposed on clinical trial investigators, a lack of control over the invention is unlikely to be found in the clinical trial context, so long as the disclosure to trial participants is limited to the drug and dose administered. The key question then, is whether the clinical trial is experimentation, or whether the clinical trial demonstrates the invention works for its intended purpose.
A Phase I clinical trial demonstrates safety, not efficacy, and so cannot generate the data needed to support a finding of public use. The FDA defines the purpose of a Phase I trial as to “evaluate the treatment’s safety, determine a safe dosage range, and identify side effects.” Moreover, the Phase I trial often tests the product in healthy subjects, negating the opportunity to generate the efficacy data needed to support a finding of public use. This was demonstrated in Eli Lilly v. Zenith (471 F.3d 1369 (Fed. Cir. 2006)) where the court found a Phase I trial of a schizophrenia drug in healthy subjects was not sufficient to invalidate the patent claiming the drug. Thus, the Phase I trial is not designed to generate the data needed to support a finding of public use, so there is no additional risk from a public use perspective in delaying the filing of the clinical trial related patent application until after the Phase I trial is completed and the Phase II trial has begun.
A Phase II clinical trial, on the other hand, can generate the data needed to support a finding of public use, so the better default timeframe for filing the clinical trial related patent application is after the Phase II trial starts. Further delaying the filing until after the Phase II trial is completed may not create additional risk as the review of the clinical trial data has been found to be part of the experimentation of the clinical trial (In re Omeprazole Patent Litig. v. Apotex Corp., 536 F.3d 1361, 1374 (Fed. Cir. 2008)).
Delaying the filing of the patent application further until after the start of the Phase III clinical trial is also possible in limited circumstances. For example, if the invention changes between the Phase II and Phase III trials, the data from the Phase III trial is needed to show the invention works for its intended purpose. This was demonstrated in In re Omeprazole where the Phase II formulation was changed prior to starting the Phase III trial by incorporating a tablet subcoating to improve stability and gastric acid resistance for the treatment of gastrointestinal disease. Accordingly, the Omeprazole court found testing of the new formulation in the Phase III trial was experimentation and not public use.
Similarly, Bayer v. Barr (2008 U.S. Dist. LEXIS 15917 (D.N.J. 2008)) involved a change in the clinical trial protocol. The invention in Bayer was a birth control formulation first tested and demonstrated to work in Europe using a homogenous patient population. A Phase III trial was subsequently conducted in the United States using a more diverse patient population representative of the United States population. The Bayer court found the testing with the more diverse patient population was necessary to determine whether the invention works in the United States, finding no public use based on the Phase III trial (Bayer, at 127-8).
The Bayer court noted, however, that “further ‘experimentation’ may constitute a barring public use” (Bayer, at 123, citing RCA Corp. v. Data Gen. Corp., 887 F.2d 1056, 1061 (Fed. Cir. 1989)). This is exemplified in Helsinn v. Teva (855 F.3d 1356 (Fed. Cir. 2017), aff’d 139 S.Ct. 628 (2019)), involving an on-sale bar. The question in Helsinn was whether the invention, a composition for treating chemotherapy-induced nausea and vomiting (CINV) containing 0.25 mg palonosetron hydrochloride, had been demonstrated to work for its intended purpose prior to the offer for sale. The Phase II trial in Helsinn demonstrated the efficacy of the 0.25 mg dose of palonosetron to treat CINV. The Phase III study investigated the same Phase II dose plus a dose of 0.75 mg. The Helsinn court found the Phase II experiments demonstrated the efficacy of the invention, distinguishing Omeprazole since there was no change in the formulation between the Phase II and Phase III trials (Helsinn, at 1375). As the 0.25 mg dose was shown to work in the Phase II trial, the Helsinn court found the invention was ready for patenting following the Phase II trial. While delaying the filing until after data is generated is advantageous, it is recommended to file the clinical trial related patent application prior to the start of the Phase III trial when there is no change in the invention or testing protocol relative to the Phase II trial.
The Risk of Clinical Trial Related Publications
Delaying the filing of the clinical trial related patent application comes with a risk that clinical trial related publications, such as the listing on clinicaltrials.gov, press releases, conference presentations, scientific papers, or even filings to the SEC, may qualify as a printed publication (35 U.S.C. § 102(a)). As with any printed publication, a lack of novelty or obviousness requires the publication, or publications, to describe the invention. Inventions such as in Omeprazole, a drug formulation having a water soluble subcoating, are not likely to be described in publications regarding the clinical trial in sufficient detail for the publication to qualify as prior art. Inventions involving a new use for a known drug, or an administration protocol involving a new dosage amount, however, are more likely to be described in even the most basic publications regarding the clinical trial and warrant an earlier filing date.
Sanofi v. Glenmark (204 F.Supp.3d 665 (D.Del. 2016)) shows the potential risk of such a publication. The publication in Sanofi described the clinical trial protocol, but not the invention, a method of decreasing a risk of cardiovascular hospitalization in a patient. In finding the patent not invalid for public use, the Sanofi court noted the investigators’ publication merely “set forth [the protocol] to test a specific hypothesis” (Sanofi, at 698). What was missing was the data demonstrating the claimed invention works for its intended purpose. Thus, mere disclosure of the clinical trial protocol was not sufficient to invalidate the asserted claims for public use where the publication did not describe the invention.
So, for most inventions and circumstances, delaying the filing of a clinical trial related patent application until after the start of a Phase II clinical trial minimizes the risk of invalidation via public use while maximizing the patent term. As shown in Omeprazole and Bayer, if there is a substantial change in the invention or testing protocol between the Phase II and Phase III trials, further delaying the filing of the clinical trial related patent application until closer to completion of the Phase III trial does not provide increased risk of invalidation for public use, and further maximizes the possible patent term. That said, consideration must be given to what the clinical trial related invention is and how it will be described in the clinical trial related publications. A more conservative filing timeline is appropriate for inventions that are more likely to be described in these publications, such as a new use for a known compound, or a dose protocol.
Best Practices for Maximizing Patent Term While Minimizing Risk of Invalidation by Public Use
- Clinical trial investigators should be subject to a strict confidentiality agreement to limit the disclosure of the invention.
- Clinical trial participants should not be informed of what the invention is, only the drug and dose being administered.
- Use the Phase II clinical trial as the starting point to determine when to file the clinical trial related patent application, with the goal of delaying the filing until after completion of the trial.
- Consider whether a change in the clinical trial formulation or the clinical trial protocol between the Phase II and Phase III trials requires the Phase III data to demonstrate the invention works for its intended purpose, thus allowing the clinical trial related patent application to be filed during or after completion of the Phase III trial.
- Consider whether any clinical trial related publications, or other factors, should accelerate the filing of the clinical trial related patent application. Merely publishing the trial protocol does not need to trigger filing the application unless the invention is the trial protocol.