Calculating Patent Term Adjustment: Part 2
 This article is second in a series focusing on various issues related to Patent Term Adjustment for U.S. patent applications. While Part 1 is a general overview of how to calculate patent term adjustment (“PTA”), this article addresses how the filing of various papers during prosecution can affect PTA. In particular, Requests for Continued Examination (RCEs), terminal disclaimers, and information disclosure statements (IDSs) can all cause adverse PTA effects if not carefully considered.
This article is second in a series focusing on various issues related to Patent Term Adjustment for U.S. patent applications. While Part 1 is a general overview of how to calculate patent term adjustment (“PTA”), this article addresses how the filing of various papers during prosecution can affect PTA. In particular, Requests for Continued Examination (RCEs), terminal disclaimers, and information disclosure statements (IDSs) can all cause adverse PTA effects if not carefully considered.
General Overview
Typically, U.S. patent term is 20 years from the earliest effective filing date, regardless of how long it takes the United States Patent and Trademark Office (“USPTO”) to examine and issue the patent. In an effort to minimize the possibility of shortened patent term, Congress created the system of PTA, codified at 35 U.S.C. § 154(b), that allows, in certain circumstances, the effective patent term to be extended past 20 years from an application’s earliest effective filing date. But as with many rules, there are exceptions.
Requests for Continued Examination
As explained in more detail in Part 1 of this blog series, 35 U.S.C. § 154(b)(1)(B) provides a guarantee of no more than a 3-year application pendency (the “3 Year Rule”). However, an exception to the 3 Year Rule includes time consumed by an RCE. It has been established that a patent application does not earn a “B” delay from the time an RCE is filed until a Notice of Allowance is issued, unless the USPTO actually resumes examination of the application after allowance. Additionally, the submission of an RCE after a Notice of Allowance has been mailed will constitute a failure to engage in reasonable efforts to conclude examination, thereby resulting in Applicant delay. Further discussion of PTA and RCEs can be found at Global IP Matters in relation to the Federal Circuit case Pfizer v. Lee and PTA rules implemented in response to Novartis v. Lee.
Example 1: B delay = 0 Days
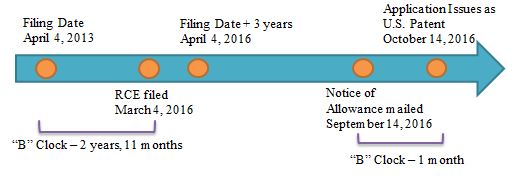
Example 2: B delay = 1 Month
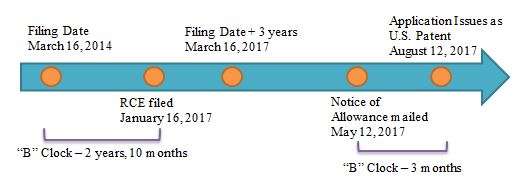
Example 3: B delay = 2 Months; Applicant Delay = 1 Month
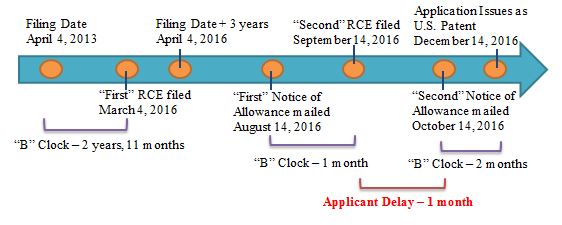
Terminal Disclaimers
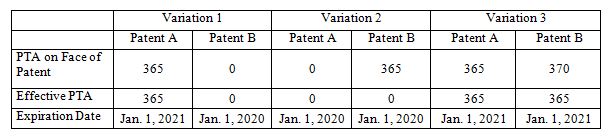
An applicant may file a terminal disclaimer to overcome a non-statutory double patenting rejection. The standard USPTO terminal disclaimer form states that the disclaimed patent will not extend beyond the term of the prior patent. While filing a terminal disclaimer to overcome such a rejection may seem innocuous, terminal disclaimers can nullify potential PTA for the disclaimed patent. In particular, PTA cannot be used to extend the term of a patent beyond the expiration date set in a filed (and approved) terminal disclaimer.
Example:
- Patent A is filed on January 1, 2000
- Patent B is a continuation of Patent A
- Terminal Disclaimer filed in Patent B over Patent A
Information Disclosure Statements
Inventors and those associated with filing or prosecuting patent applications as defined in 37 CFR § 1.56 have a duty to disclose to the USPTO information that is material to patentability. This duty is deemed satisfied if such information is submitted to the USPTO via an IDS. While 37 C.F.R. § 1.97 generally governs when and how IDSs may be submitted at different stages of prosecution, a “timely” filed IDS may not be so timely for purposes of PTA.
As set forth in 37 C.F.R. § 1.704, the filing of an IDS can result in a reduction of the PTA under several circumstances, for example when the IDS is filed after a response to an office action has been filed. Therefore, the filing of an IDS under certain circumstances can trigger significant PTA reductions under 37 C.F.R. § 1.704.
Example: An RCE was filed on March 24, 2016, and no subsequent action taken by USPTO before the filing of an IDS. Although the IDS filing is considered “timely” under 37 CFR § 1.97, the IDS filing constitutes a supplemental reply under 37 C.F.R. § 1.704, thereby resulting in an accrual of 364 days of Applicant delay.

The “30-Day Statement” Exception
A reduction of PTA can be avoided for an IDS filing by filing a statement under 37 C.F.R. § 1.704(d), which is provided below with emphasis added. A statement under 37 C.F.R. § 1.704(d) (the “30-Day Statement”) should not be confused with the certification statements under 37 C.F.R. § 1.97(e). Unlike the statements under 37 C.F.R. § 1.704(d), the statements under 37 C.F.R. § 1.97(e) have no effect on preventing PTA reduction.
37 C.F.R. § 1.704(d) states
“A paper containing only an [IDS] … will not be considered a failure to engage in reasonable efforts to conclude prosecution … under paragraphs (c)(6), (c)(8), (c)(9), or (c)(10) of this section … if the paper … is accompanied by a statement that each item of information contained in the [IDS] … (i) Was first cited in any communication from a patent office in a counterpart [1] foreign or international application or [2] from the Office, and this communication was not received by any individual designated in 1.56(c) more than thirty days prior to the filing of the [IDS]…; or (ii) Is a communication that was issued by a [1] patent office in a counterpart foreign or international application or [2] by the Office, and this communication was not received by any individual designated in 1.56(c) more than thirty days prior to the filing of the information disclosure statement.”
Example: An RCE was filed on March 24, 2016, and no subsequent action taken by USPTO before the filing of an IDS with a 30-Day Statement. Thus, no Applicant delay.

Conclusion
While the above discussion provides a general overview of certain factors that can affect PTA and how it is calculated, patentees are encouraged to consider when and whether to file various papers under every application’s particular circumstances and to verify PTA calculations according to the current rules and regulations of the USPTO.

Calculating Patent Term Adjustment: Part 1
June 22, 2017| Blog

Patent Term Adjustment: Lessons Learned from the Federal Circuit Decision in Actelion v. Matal
February 14, 2018| Blog

Federal Circuit Decision Impacts Patent Term Adjustment Calculation
February 7, 2014| Blog
Authors


